Abstract
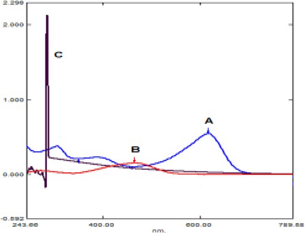
A simple, rapid and low-cost spectrophotometric method for determination of Methyldopa (MDA) based on ion-pair formation using Bromothymol blue (BTB) as a reagent in alkaline medium (pH 8.7). The absorbance of the green-blue-coloured product is measured at 616 nm. Beer's Law is obeyed at concentration range up to 5-20μg/ml with molar absorptivity 0.8279x104 L/mol.cm. The correlation coefficient, limit of detection and limit of quantification were 0.9982, 0.4318 μg/ml and 1.4393 μg/ml respectively. The method has been successfully applied to the determination of Methyldopa in pharmaceutical preparations.
Full text article
Generated from XML file
Authors
Khalaf F Al Samarrai, Eman Thiab A Al Samarrai, & Baidaa Adnan Al Samarrai. (2019). Spectrophotometric determination of methyldopa in pharmaceutical preparation via ion pair formation. International Journal of Research in Pharmaceutical Sciences, 10(2), 1367–1371. Retrieved from https://ijrps.com/home/article/view/3871

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.