Abstract
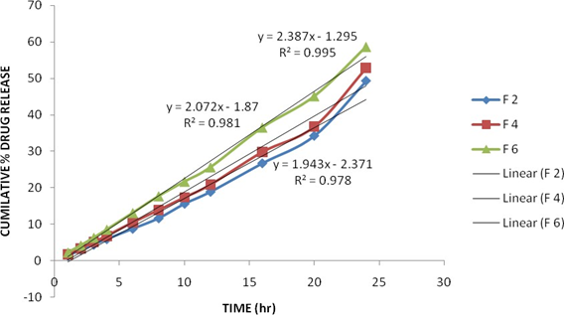
The main objective of the work was encapsulating of Doxorubicin hydrochloride in liposomal formulation for treatment of cancer chemotherapy. Conventional compositions of Doxorubicin hydrochloride were available as freeze-dried product or as a solution of doxorubicin hydrochloride in water. Both these products have been associated with a number of toxicities when administered intravenously. To overcome these problems, in the present study, inclusion of Doxorubicin hydrochloride in liposomal formulation has proved to be good approach to eliminate the toxicities and improve drug antitumor activity. In this study, Doxorubicin hydrochloride liposomes containing Hydrogenated Soy Phosphatidyl Choline, Cholesterol, various stabilizers and ammonium sulphate prepared by dried thin film hydration method. The characterization of liposomes was carried out by vesicle size, zeta potential, %free drug and in-vitro dissolution. The release kinetics of formulations containing neural, negative and positive stabilizers followed zero-order release kinetics. Hence it could be concluded that stabilizers like Stearylamine and Phosphotidyl glycerol along with Hydrogenated Soy Phosphatidyl Choline (HSPC) and Cholesterol were suitable carriers for the preparation of Doxorubicin HCl liposomes.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.