Abstract
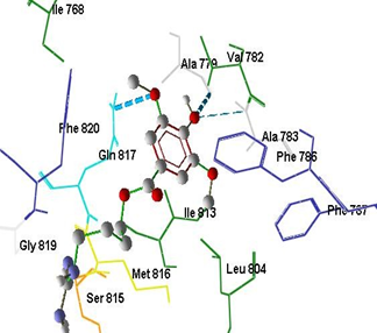
Leonurine is an alkaloid produced from the medicinal plant of deungdeureuman (Leonurus artemisia L.). It has been reported to show antiapoptotis, antihipertension, antiinflamation, antipiretic, and diuretic effect. In addition was also reported to show despile different meaning of aphrodisiac effect and evection, its interessting to study the possible influence of leonurine and its derivatives on erectile dysfunction condition. The cryatalographic structure of this enzyme is published and can be obtained from Brookhaven Data Bank with the code of PDB ID : 2H42. Applying this PDB data, it is posible to perfom docking study of leonurine and its derivatives on PDE-5. As well as the pharmacokinetic properties of those compounds including oral absorption, distribution, metabolism, and toxicity (ADME/T) by means of in silico method. Thirteen Leonurine derivatives design which produced by stuctural modification of leonurine, especially on their carboxylic and methoxy group, were included in this research. The affinities of those compound designs were studied applying molecular docking using ArgusLab 4.0.1 2004 program, while those of pharmacokinetic properties were performed by PreADMET online free program. The result showed that ten leonurine derivatives designs have lower free energy binding (-9.460 kcal/mol) in comparison to leonurine (-7.397 kcal/mol). This compound has human intestial absorption (HIA), Cac0-2 cell permeability, and plasma protein binding values of 18.71%, 8.28 (nm/Sec), and 66.79 %, respectively, which are comparable to those of leonurine and other leonurine derivatives design. Based on overall results, it was conclude that 4-amino buthyl -4(-3amino-5-hydroxypentyl)guanidino)3,5-dimethoxybenzoat was an leonurine derivative design with highest possibility to be further developed as a potential PDE5 inhibitor.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.