Abstract
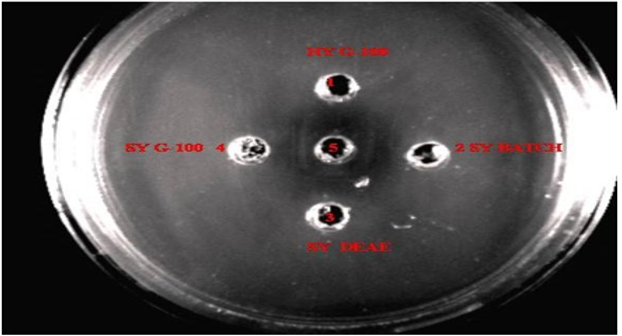
Riboflavin carrier protein has been isolated and purified from the Indian spotted owlet (Athene brama). Purification could be achieved by DEAE-Sepharose column chromatography and gel filtration chromatography on Sephadex G-100. Further the RCP was immunologically characterized and compared with the hen (Gallus gallus domesticus) egg yolk RCP (RfBP). The protein was characterized using absorption, fluorescence and CD spectral analysis. Comparison of the mobility of the purified proteins with the standard molecular weight marker proteins revealed that the spotted Owlet egg RCP had a molecular weight close to 29.2kDa and it was approximately 3 kDa less than the hen egg Yolk RCP.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.