Abstract
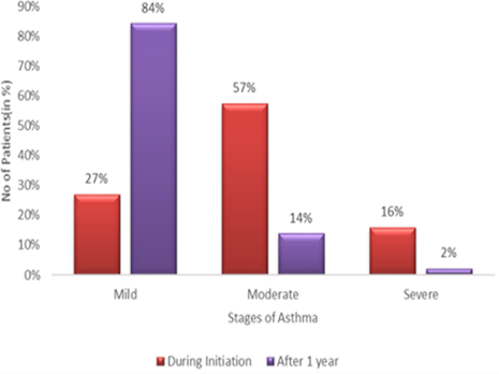
The purpose is to study the asthma symptom improvement and efficacy of formoterol (LABA) and Budesonide (ICS) combination in the asthma management in northern districts of Tamilnadu. It is a multicentric, non-comparative, questionnaire and random sampling study was conducted in 145 mild to severe asthmatic patients. They were in Formoterol plus budesonide combination inhalation drugs available in DPI and pMDI. Among the 145 asthmatic patients, 45 patients, 6 patients and 94 patients were in DPI, nebuliser and pMDI devices respectively. During drug initiation, The Asthmatics were in mild (27%), Moderate (57%) and Severe (16%) stages. After one year of follow-up, the number of patients in the mild is 84%, moderate 14% and severe 2%. Among 45 DPI Asthmatic patients, 29 patients, 15 patients and 1 patient have reported the handling the devices as easy, medium and hard respectively. On the other hand, 94 pMDI asthmatic patients, 38 patients, 48 patients and 8 patients have reported the handling of devices as easy, medium and hard respectively. The treatment resulted in 77 patients as good, 65 as satisfactory and 3 as same. After one year, all the 145 asthmatic patients adhered with the treatment and experienced symptom improvement with 53% patients as good, 45% patients as satisfactory and 2% patients as same. The treatment of formoterol and budesonide combination in the northern districts of Tamilnadu have effectively controlled the asthmatic symptoms and improved the quality of life in asthmatics. Moreover, the patient’s adherence to the treatment is good in the northern parts of Tamilnadu.
Full text article
References
Bateman, E. D., Reddel, H. K., O’Byrne, P. M., Barnes, P. J., Zhong, N., Keen, C., Jorup, C., Lamarca, R., Siwek-Posluszna, A., FitzGerald, J. M. 2018. As Needed Budesonide–Formoterol versus Maintenance Budesonide in Mild Asthma. New England Journal of Medicine, 378(20):1877–1887.
Beasley, R., Holliday, M., Reddel, H. K., Braithwaite, I., Ebmeier, S., Hancox, R. J., Harrison, T., Houghton, C., Oldfield, K., Papi, A., Pavord, I. D., Williams, M., Weatherall, M. 2019. Controlled Trial of Budesonide–Formoterol as Needed for Mild Asthma. New England Journal of Medicine, 380(21):2020–2030.
Beasley, R., Pavord, I., Papi, A., Reddel, H. K., Harrison, T., Marks, G. B., Hancox, R. J., Weatherall, M. 2016. Description of a randomised controlled trial of inhaled corticosteroid/fast-onset LABA reliever therapy in mild asthma. European Respiratory Journal, 47(3):981–984.
Byrne, P. M. O., Fitzgerald, J. M., Bateman, E. D. 2018. Inhaled combined budesonide formoterol as needed in mild asthma. N Engl J Med, 378(20):1865–1876.
Chen, Q., Hu, C., Yu, H., Shen, K., Assam, P. N., Gillen, M., Liu, Y., Dorinsky, P. 2019. Pharmacokinetics and Tolerability of Budesonide/Glycopyrronium/Formoterol Fumarate Dihydrate and Glycopyrronium/Formoterol Fumarate Dihydrate Metered Dose Inhalers in Healthy Chinese Adults: A Randomized, Double blind, Parallel-group Study. Clinical Therapeutics, 41(5):897–909.e1.
Dhandayuthapani, M., Shivashankar, M. 2020. Asthmatics Treatment Updation and Compliance in the Asthma Management in Northern Districts of Tamilnadu. International Journal of Research in Pharmaceutical Sciences, 11(3):4990–4997.
Du, W., Zhou, L., Ni, Y., Yu, Y., Wu, F., Sh, G. 2017. Inhaled corticosteroids improve lung function, airway hyperresponsiveness and airway inflammation but not symptom control in patients with mild intermittent asthma: A meta-analysis. Exp. Ther. Med, 14(2):1594–1608.
Ekberg-Jansson, A., Svenningsson, I., Rågdell, P., Stratelis, G., Telg, G., Thuresson, M., Nilsson, F. 2015. Budesonide inhaler device switch patterns in an asthma population in Swedish clinical practice (ASSURE). International Journal of Clinical Practice, 69(10):1171–1178.
Ferguson, G. T., Papi, A., Anzueto, A., Kerwin, E. M., Cappelletti, C., Duncan, E. A., Nyberg, J., Dorinsky, P. 2018. Budesonide/formoterol MDI with co-suspension delivery technology in COPD: the TELOS study. European Respiratory Journal, 52(3):1801334–1801334.
Galffy, G., Mezei, G., Nemeth, G., Tamasi, L., Muller, V., Selroos, O. 2013. Inhaler competence and patient satisfaction with Easyhaler: results of two real-life multicentre studies in asthma and COPD. Drugs RD, 13(3):215–237.
Haikarainen, J., Selroos, O., Löytänä, T., Metsärinne, S., Happonen, A., Rytilä, P. 2017. Budesonide/Formoterol Easyhaler®: Performance Under Simulated Real-Life Conditions. Pulmonary Therapy, 3(1):125–138.
Hantulik, P., Wittig, K., Henschel, Y., Ochse, J., Vahteristo, M., Rytila, P. 2015. Usage and usability of one dry powder inhaler compared to other inhalers at therapy start: an open, non-interventional observational study in Poland and Germany. Pneumonologia i Alergologia Polska, 83(5):365–377.
Husereau, D., Drummond, M., Petrou, S., Carswell, C., Moher, D., Greenberg, D., Augustovski, F., Briggs, A. H., Mauskopf, J., Loder, E. 2013. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ, 346(mar25 1):f1049– f1049.
Lähelmä, S., Vahteristo, M., Metev, H., Taseva, M., Stamatova, N., Bartha, A., Schlezák, J., Sairanen, U. 2016. Equivalent bronchodilation with budesonide/formoterol combination via Easyhaler and Turbuhaler in patients with asthma. Respiratory Medicine, 120:31–35.
Malmberg, L. P., Everard, M. L., Haikarainen, J., Lähelmä, S. 2014. Evaluation of In Vitro and In Vivo Flow Rate Dependency of Budesonide/Formoterol Easyhaler®. Journal of Aerosol Medicine and Pulmonary Drug Delivery, 27(5):329–340.
Mckeever, T., Mortimer, K., Wilson, A. 2018. Quadrupling the inhaled glucocorticoid dose to abort asthma exacerbations. N Engl J Med, 378(10):902– 912.
Nunes, C., Pereira, A. M., Morais-Almeida, M. 2017. Asthma costs and social impact. Asthma Research and Practice, 3(1):3–3.
O’Byrne, P. M., Jenkins, C., Bateman, E. D. 2017. The paradoxes of asthma management: time for a new approach? European Respiratory Journal, 50(3):1701103–1701103.
Pirożyński, M., Hantulik, P., Almgren-Rachtan, A., Chudek, J. 2017. Evaluation of the Efficiency of Single-Inhaler Combination Therapy with Budesonide/Formoterol Fumarate in Patients with Bronchial Asthma in Daily Clinical Practice. Advances in Therapy, 34(12):2648–2660.
Price, D., Fletcher, M., van der Molen, T. 2014. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. Primary Care Respiratory Medicine, 24(1):14009– 14009.
Reddel, H. K., Busse, W. W., Pedersen, S., Tan, W. C., Chen, Y.-Z., Jorup, C., Lythgoe, D., O’Byrne, P. M. 2017. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. The Lancet, 389(10065):157–166.
Slejko, J. F., Ghushchyan, V. H., Sucher, B., Globe, D. R., Lin, S.-L., Globe, G., Sullivan, P. W. 2014. Asthma control in the United States, 2008-2010: Indicators of poor asthma control. Journal of Allergy and Clinical Immunology, 133(6):1579–1587.
Stallberg, B., Lisspers, K., Hasselgren, M., Janson, C., Johansson, G., Svardsudd, K. 2009. Asthma control in primary care in Sweden: a comparison between 2001 and 2005. Prim Care Respir journal, 18(4):279–286.
Subramanian, N., Kumaresan, C. 2012. Dry powder inhaler formulation aspects. Pharma times, 44:14–18.
Tamasi, L., Szilasi, M., Galffy, G. 2018. Clinical Effectiveness of budesonide/formoterol fumarate Easyhaler for patients with poorly controlled obstructive airway disease: a real-world study of patient-reported outcomes. Adv Ther, 35(8):1140–52.
Virchow, J. C., Crompton, G. K., Negro, R. D., Pedersen, S., Magnan, A., Seidenberg, J., Barnes, P. J. 2008. Importance of inhaler devices in the management of airway disease. Respiratory Medicine, 102(1):10–19.
Wang, X., Li, Q., Liu, R., He, J., Wu, D., Wang, Y., Zhang, J. 2018. ADRB2 Arg16Gly polymorphism and pulmonary function response of inhaled corticosteroids plus long-acting beta-agonists for Asthma treatment: A Systematic review and meta-analysis. Can. Respir. J, pages 5712805–5712805.
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.