Abstract
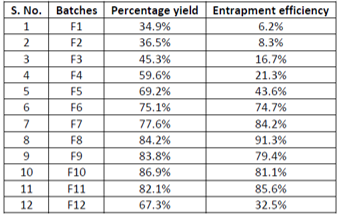
The present investigation was aimed at developing Bendamustine-loaded poly (L-lactide) (PLA) based biodegrada- ble microspheres by a double emulsion solvent evaporation technique which would have sustained release of the drug. The poly (L-lactide) (PLA) microspheres containing Bendamustine as a drug and evaluate the various physicochemical characteristics of the formulations, namely morphology, particle size, FTIR, Bendamustine encapsulation efficiency and In vitro Bendamustine release profile. Bendamustine-loaded microspheres were prepared by double emulsion solvent evaporation method with different Bendamustine, PLA ratios and at different speeds of homogenization keeping the amount of Bendamustine constant in all the formulations and different amount of salt (NaCl) concentrations Accelerated stability testing was performed with the optimized formulations for a period of two months. The mean particle size and encapsulation efficiency of the microspheres were found to decrease as the speed of homogenization increased and the encapsulation efficiency was increased with increase in salt (NaCl) concentration. The in vitro release study showed a slow and steady release pattern of Bendamustine. Thus a sustained release formulation of Bendamustine loaded PLA microspheres were developed.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.