Abstract
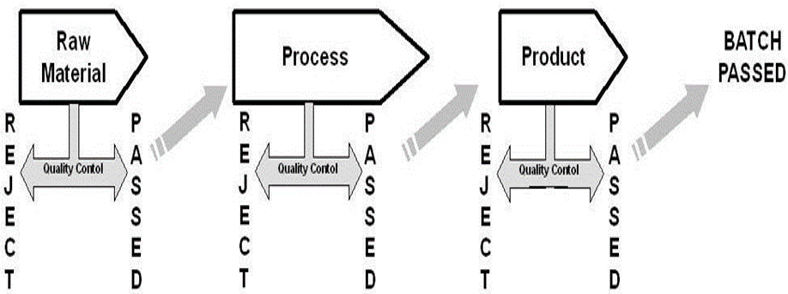
Process Analytical Technology (PAT) is an initiative of US FDA to ensure for a better quality product with minimum cost, minimum wastage and defects by a constant check on the quality attributes throughout a process. The PAT concept proved beneficial in maintaining the quality of many of the pharmaceutical and biotechnological process. Yet for all there had been much opposition on implementation of PAT. Fermentation being one of the prime processes linking to most of the pharmaceutical products proves to be an excellent stand on this issue if at all PAT is successful in its case. Of the many techniques suggested by PAT for analyzing the quality of the process Green Fluorescent Protein (GFP), a step ahead in process analysis. The review aims at supporting the PAT concept with the discussion made on the benefits of GFP in various fermentation processes.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.