Abstract
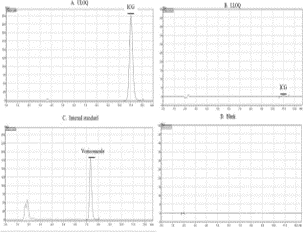
A robust and economical assay for routine determination of indocyanine green pharmacokinetics was developed and validated using high-performance liquid chromatography with a photodiode array detector. Plasma specimens from critically ill patients and those with hepatitis on various comedications were used as blanks for validation of this assay. Extraction of indocyanine green was performed by simple protein precipitation with acetonitrile, and the supernatant was separated using an octadecyl column with detection at 784 nm. Blanks were found to have no interference for 40 blanks of patients who were on 56 different medications. The precision for LLOQ (0.5 µg/ml) as determined by the percentage coefficient of variation was 1.19. Stability of plasma calibration standards and stock were determined over a period of 61 days, and ICG was found to be stable for 20 days. Stability of whole blood specimens containing ICG was determined at 4°C for a period of 4 hours.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.