Abstract
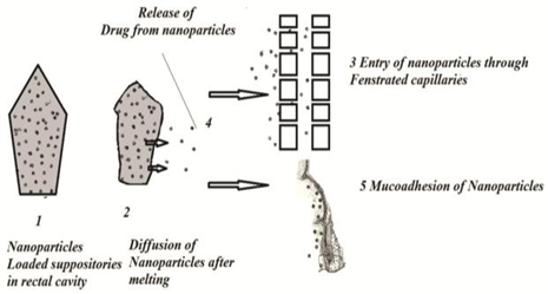
Aspirin suppositories are in commercial use suffer with side effects such as irritation, burning sensation, rectal hemorrhage. The aim of the present work is to formulate aspirin nanoparticles loaded suppositories and to perform In vitro investigation for the prepared suppositories. Initially aspirin-chitosan nanoparticles were prepared by ionic gelation method and the nanoparticles evaluated for different In vitro evaluation studies; based on results the best formulation was selected and in order to know the diffusion efficiency, different compositions of aspirin glycerogelatin suppositories were prepared and subjected to various In vitro evaluation studies and best composition was selected. From the previously performed evaluation studies best formulation from aspirin nanoparticles incorporated in to selected glycerol gelatin bases and evaluated for In vitro characteristics. The results indicates that formulation Fa9 Aspirin nanoparticles were proved to be best formulation with 88.3±1.1 % of drug release at the end of 24hr, with zero drug release. In vitro characterization performed for aspirin suppositories indicates that Fs2, Fs4, Fs9 and Fa11 was proved to be best composition with highest percentage of drug release at the end of 60 minutes with 98.06±1, 99.3±0.45, 97.6±1.8 and 97±1 drug release and other characteristic studies performed indicates that all formulation are ideal characteristics. Previously selected bases composition used for the loading of nanoparticles based on displacement value results indicates that drug release appears with a lag phase initially and controlled for a period of 24hr.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.