Abstract
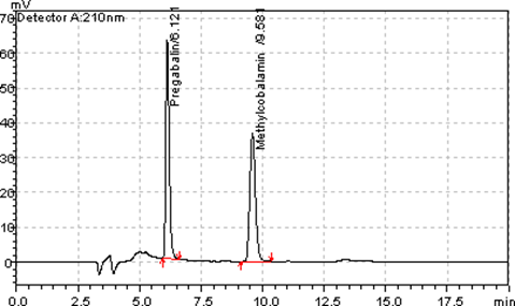
A simple, precise and accurate RP- HPLC method was developed and validated for the estimation of pregabalin and methylcobalamin in capsule formulation. The HPLC instrument used was Shimadzu Prominence LC20AT with UV detection. Inertsil ODS 3 C-18 column with dimensions of 250 mm length, particle size of 3 microns and internal diameter of 4.0 mm was used for separation. The mobile phase consisted of 60 volumes of buffer 0.01 M potassium dihydrogen and 0.01M dipotassium hydrogen phosphate and 40 volumes of solvent methanol at a flow rate of 0.6 ml/min. The wavelength of 210 nm was used for the detection. The method was validated for linearity, accuracy, precision, LOD, LOQ and robustness as per ICH guidelines. The response was found to be linear for the concentration range of 75µg/ml to 1125µg/ml for pregabalin and 0.75µg/ml to 11.25µg/ml for methylcobalamin. The limit of detection and limit of quantification was 5µg/ml and 15µg/ml for pregabalin and 0.05µg/ml and 0.15µg/ml for methylcobalamin. This validated method was applied for the estimation of pregabalin and methylcobalamin in commercial capsule formulation.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.