Abstract
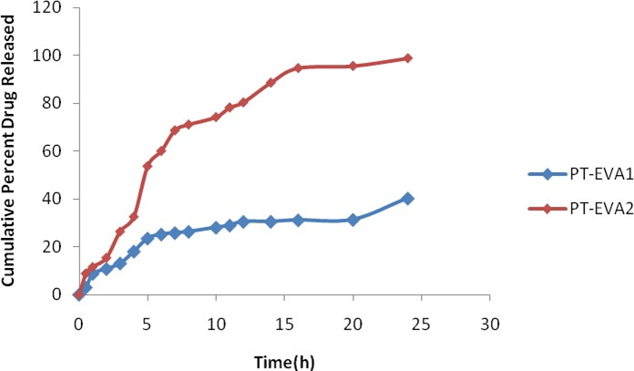
The aim of the present investigation was to enhance the solubility and impart a controlled release pioglitazone - βCD matrix tablets; pioglitazone is an oral hypoglycemic agent, which belongs to Class II of BCS with relatively short elimination half life. Inclusion complex of pioglitazone with β-cyclodextrin was prepared by kneading, co- precipitation, physical mixture and evaluated for its in-vitro release. The dissolution study of kneading complex shows significant increase in the drug release from kneading complex than pure drug and physical mixture. Matrix tablet complex equivalent to 30 mg pioglitazone were prepared by using ethylene vinyl acetate (EVA), ethyl cellulose (EC) and starch acetate (SA) evaluated for various tablet properties and in-vitro dissolution studies. The feasibility of employing βCD complexes in the design of controlled release matrix tablets of pioglitazone for obtaining slow controlled and complete drug release in 24 h.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.