Abstract
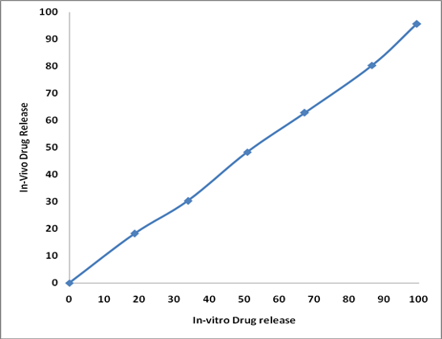
Administration of conventional tablets of atenolol has been reported to exhibit fluctuations in the plasma drug levels, resulting either in manifestation of side effects such as diarrhea, ischemic colitis and mesenteric arterial thrombosis or reduction in drug concentration at the receptor site. Transdermal drug delivery (TDD) method has been selected as it provides controlled release of the drug, and produces a steady blood-level profile, leading to reduced systemic side effects and, sometimes, improved efficacy over other dosage forms. In the present work efforts have been made to prepare transdermal drug delivery system of Atenolol using various blends of polymers such as Eudragit RS 100, Hydroxy Propyl methyl cellulose and Poly vinyl pyrrolidone using propylene glycol as a permeation enhancer. The prepared patches were evaluated for various physicochemical properties and in-vitro drug release studies. The release were fitted to statistical treatment such as Zero order kinetics, Higuchi’s and Peppa’s plot. The optimized formulation from the in-vitro drug release study is used to carry out in-vitro skin permeation study using porcine ear skin, snake shed skin and rat skin. The in-vivo evaluation of formulation F 7 (2% Eudragit RS 100, 1%HPMC) show better correlation with the in-vitro drug release which confirms the achievement of targets of the present study such as controlled prolonged zero order release, reduced frequency of administration, greater therapeutic effect, overcome the side effects, simplify the treatment regimen and thus may improve patient compliance.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.