Abstract
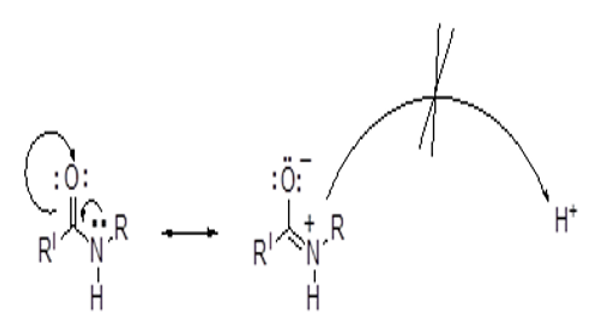
A simple, validated and affordable visible spectrophotometric method was developed for the determination of piperacillin present in bulk and tablet formulation. The proposed method involves the development of a coloured ionassociation complex between piperacillin cation and tropaeolin-ooo anion, which can be further extracted in chloroform. In the formation of cation from piperacillin, site of protonation is oxygen of amide group. Two secondary amides of piperacillin are protonated to form a doubly charged cation. Maximum absorption was observed at 529 nm for the coloured complex. Regression analysis (r = 0.9999) shows that the plotted calibration curve exhibits good linearity in the studied range of concentration (4.0–24.0 μg mL-1). As per the existing guidelines of ICH, various parameters (interday precision, intraday precision, accuracy, ruggedness, LOD, LOQ) of the method were tested for validation. Low values of relative standard deviation (< 2 %) were observed indicating that the proposed method is reproducible, accurate and precise. The proposed method was extended to assay piperacillin powder for injection formulation.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.