Abstract
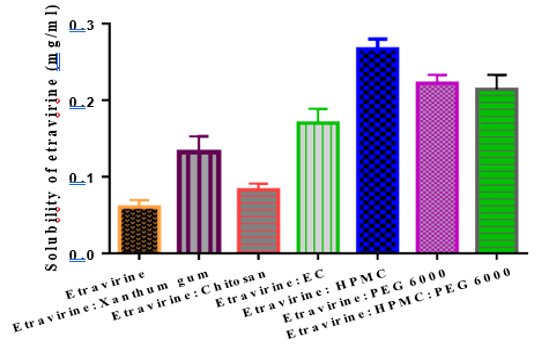
Solid dispersions (SDs) technique represents a promising approach to enhance the solubility, dissolution rate and bioavailability of poorly water-soluble drugs. Etravirine is a new non-nucleoside reverse transcriptase inhibitor (NNRTI) of human immune deficiency (HIV) virus 1 and it belongs to class IV in Biopharmaceutics classifications system (BCS). The major problem with this drug is its poor solubility in biological fluids, which results in poor bioavailability after oral administration. In the present study, five formulations of etravirine solid dispersion were formulated by solvent evaporation technique using Hydroxypropyl methylcellulose (HPMC), Polyethylene glycol (PEG 6000) as hydrophilic carriers with the ratio of 1:1 and 1:2. The dissolution result shown that there was a significant increase in the solubility of etravirine from all formulations. It was observed that formulation (SD2) comprising Etravirine: HPMC (1:2) ratio has shown enhanced solubility and faster dissolution rate. This may due to the conversion of crystalline to an amorphous form of etravirine in solid dispersion consists of a hydrophilic carrier and also increase in wettability. Hence the study was concluded that etravirine SDs could be beneficial for the treatment of HIV/AIDS with enhanced dissolution rate and bioavailability.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.