Abstract
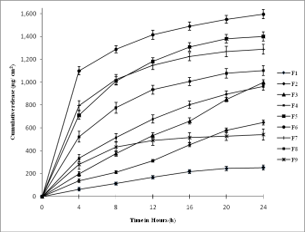
The present work was intended to design alternative dosage form to the conventional tablets of the Buspirone hydrochloride (BH); as about 96% drug is metabolized during its first pass by oral route. An adhesive matrix system of BH was prepared by taking different ratios of Polyvinylpyrrolidone K30 (PVP) and Hydroxypropyl methylcellu- lose LMV (HPMC) with polyethylene glycol 400 (PEG) as plasticizer. Oleic acid was incorporated as penetration enhancer and sodium lauryl sulfate (SLS) as solubilizer in the hydroalcoholic solution. Resultant solution was dried on the Polyvinyl Alcohol EF (PVA) backing membrane to prepare adhesive matrix type transdermal patch. Physical evaluation of the adhesive matrix layers obtained by the various combinations of PVP and HPMC were carried out by performing thickness uniformity, ultimate tensile strength, peel adhesion, ball adhesion and moisture absorp- tion studies. Permeation studies were performed using Keshary-Chien diffusion cell through human cadaver skin in 10% buffered formalin (7.4pH). Approximate zero order release kinetics was observed when a patch containing 4 mg/cm2 of BH created a flux of 42µg/cm2h-1 and penetrated about 1mg/cm2 of drug in a day. Transdermal patch of BH provides a superior option to conventional tablets and creates reasonable change in the drug therapy of anxiety patients.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.