Abstract
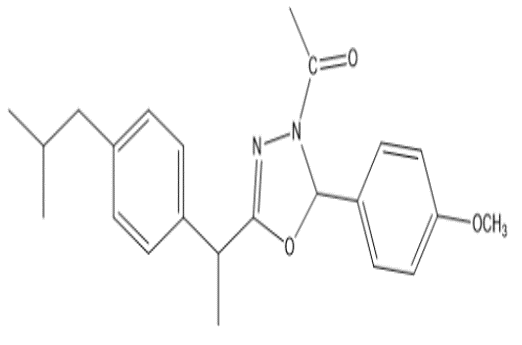
This work implicates the synthesis of Ibuprofen Acyl Hydrazones and then Converted into the new 1,3,4-Oxadiazoline derivatives that characterized by proton-NMR, FT.IR and elemental microanalysis (CHN) techniques. The intermediates and final compounds were investigated for their physicochemical properties, including the melting point, color, the yield percent, and thin-layer chromatography performed by using TLC silica gel (60) F254, Merck (Germany), to identify the purity of the products and to know the reaction endpoint. Compounds were monitored by UV light irradiation and the elution by using the following systems:: ethyl acetate: hexane ( 3:7), ethyl acetate, ethanol:dioxan (1:1) and methanol: chloroform (1:9). The study was performed using Swiss albino mice (25-30 g) for the pharmacological activity assessment. Hind edema template of carrageenan used for anti-inflammatory activity assessment and the analgesic activity evaluated using ( writhing induced by acetic acid ) and hot plate method, the results show that all the final compounds present with good anti-inflammatory plus analgesic activities exhibited in the animal model of our experimental work , we observed that the standard compound and the synthesized derivatives substantially reduced carrageenan-induced edema at all-times (2,4,6,24) hours, all chemically synthesized new compounds actually significantly reduced the number of acetic acid writhings induced in mice and finally in hot plate method there is high increase in the reaction time to painful stimulation.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.