Abstract
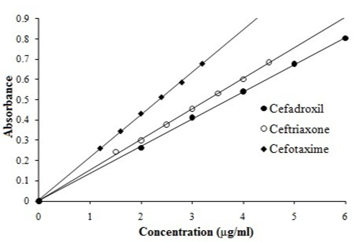
Selective and highly sensitive spectrophotometric method was developed for the determination of three cephalosporins, namely cefadroxil, ceftriaxone and cefotaxime. Spectrophotometric method involves adding a measured excess of NBS to the drugs in acid medium followed by determination of residual NBS by reacting with a fixed amount of methyl orange and measuring the absorbance at 508nm. The measured absorbance is found to increase linearly with the concentration of cephalosporins serving as basis for quantitation. Under the described conditions, the proposed method is linear over the concentration range of 2.0-6.0 µg/ml, 1.5-4.5 µg/ml and 1.2-3.2 µg/ml for cefadroxil, ceftriaxone and cefotaxime respectively and the coefficients of variation was found to be in the range of 0.9992-0.9997. The recoveries of the title compounds in spiked plasma and in pharmaceutical dosage form ranged from 83.0 to 118.0% with a limit of detection (LOD) in the range of 0.0240- 0.088 µg/ml and limit of quantification in the range of 0.0720-0.264 µg/ml (LOQ) of for all the three drugs.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.