Abstract
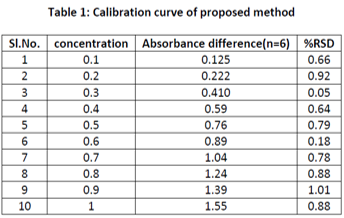
A simple, precise and accurate difference spectroscopic method has been developed for the estimation of aspirin in pharmaceutical dosage form. The proposed method is based on the principle that aspirin can exhibit two different chemical forms which differ in absorption spectra in basic and acidic medium. Science the drug was not freely soluble in the distilled water, 1M sodium salicylate has been used as hydrotropic solubilising agent for aspirin to carryout its spectrophotometric analysis and make the process cost effective. The stock solution was prepared by taking water as solvent. Further dilutions were made by using 0.1M sodium hydroxide and 0.1M hydrochloric acid separately. The maxima and minima in the difference spectra of aspirin were 312nm and 285nm, respectively. Difference in absorbance between maxima and minima was calculated to find out the amplitude. The amplitude was plotted against concentration. Beer’s-Lambert’s law is valid in the concentration range 0.1-1μg/ml. The result of analysis was validated statistically and by recovery study.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.