Abstract
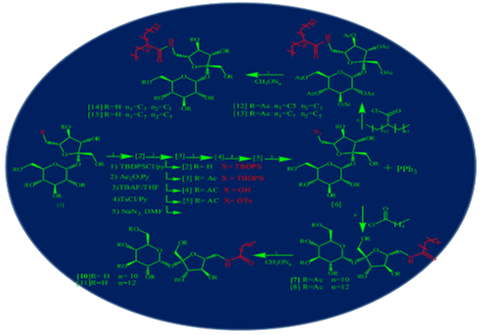
A series of four new derivatives of sucrose have been synthesized using the straightforward methodology in order to give a mono substituted analogs of sucrose at C-6’ of fructose moiety. The synthesis was started from the reaction of sucrose with tert-butylchlorodiphenylsilane, which is able to react with an only less steric hindrance hydroxyl group at C-6’ due to its bulky structure. The other hydroxyl groups were acetylated by the reaction with acetic anhydride in pyridine. Then free the hydroxyl group at C-6’ again by the treatment with t-butylammonium fluoride in THF. The later was activated by conversion to a good leaving group via tosylation, followed by functionalized via azidation to give the precursor of the target series hepta-O-acetyl-6’-azido- sucrose. The precursor was coupled with four alkyl halides (C12, C8-4, C14, C10-8) via Staudinger reaction to produced the target structure after deacetylating. The purity and chemical structure of the synthesized compound was confirmed by CHN elemental analysis, high-resolution mass and 1H, 13C NMR spectroscopy.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.