Abstract
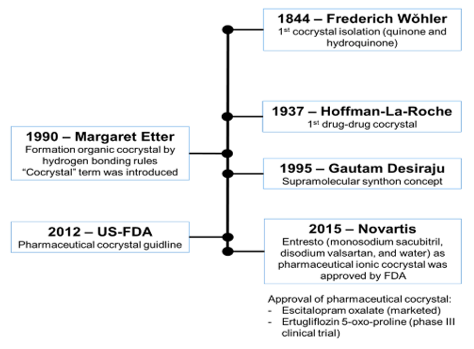
Nowadays, most of marketed APIs are in the salt form. But not every single API was ionizable. Thus cocrystallization technique arised to overcome the problem. The aim of this review is to compare the solubility profile of salt and cocrystal. Both salt or cocrystal were used to improve the solubility of APIs and claimed for each other, which is the most effective in solubility enhancement. The salt was sformed by ionic interaction, whilst cocrystal formed by hydrogen. Both interactions changed the interaction energy and crystal packing, thus changing the physicochemical properties of APIs. Besides, other factors affecting the solubility of the binary system were melting point, particle morphology, and solubility of coformer. The solubility comparison led to understand the underlying mechanism on solubility enhancement of salt and cocrystal itself.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.