Abstract
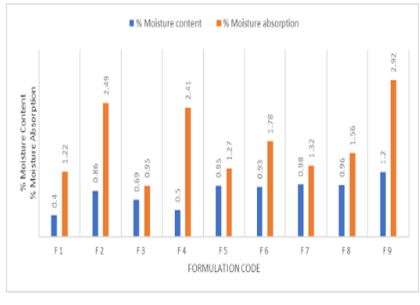
The main aim of this study was to expand the present formulation with patch and to evaluate the transdermal patches of Flurbiprofen, an NSAID (Non-Steroidal Anti-Inflammatory Drug) used in the treatment of arthritis. Flurbiprofen is required at a sustained rate of its short half- life (3-4 hours) long term percutaneous absorption is required for its gastrointestinal side effects. Hence in this study, an effort was done to develop transdermal patches of Flurbiprofen by employing a different combination of polymers were prepared by the solvent evaporation technique. Nine formulations were prepared consists of Hydroxy Propyl Methyl Cellulose (HPMC) E15 and polyvinylpyrrolidone in the ratios of 1:1, 1:2. Formulation, HPMC E15 and Ethylcellulose (EC) in 1:1, 1:2. Formulations, HPMC E15 and Eudragit RS100 in 1:1, 1:2, and Formulation HPMC E15 and Eudragit RL100 in 1:1, 1:2. Formulations (F1- F8). F9 formulations contain HPMC E15, Eudragit RS100 in 2:1 ratio with permeation enhancer DMSO 20% v/w of propylene glycol was used as a plasticizer in all the formulations. The developed patches were considered for several physicochemical parameters. In vitro drug release studies showed maximum percentage in 24 hrs for F9 formulation 94.31±1.43, showed maximum Ex-vivo skin permeation of 10,120±0.91 µg/cm2, and the obtained flux meets the required flux. The resultant data was fitted into zero; first, Higuchi and Peppas model and Formulation F9 followed zero order with R2 0.972. Drug compatibility studies were resulted by FTIR. The results specify that Flurbiprofen transdermal patch can be designed with the required amount of flux with desire mechanical properties for the cause of better therapeutic benefits.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.