Abstract
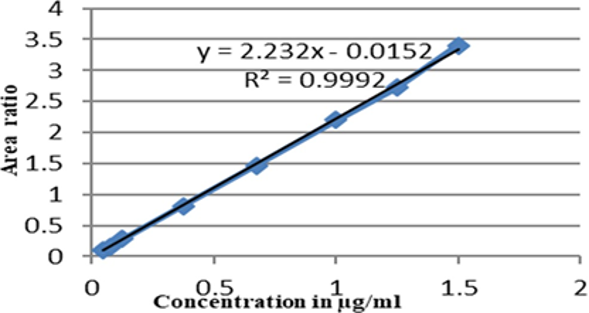
New stability-indicating RP-UPLC technique was developed for the quantification of ertugliflozin and metformin in human plasma and validated as per the regulatory guidelines. Both the drug components and internal standard were spiked to blank plasma and subjected to liquid-liquid extraction with the mobile phase. The resultant solution was infused into Acquity BEH-C18 (1.7 µ, × 100 2.1mm) non-polar column comprising NaH2PO4 buffer (pH-3.5), methanol and acetonitrile in the ratio of 50:10:40% v/v/v as mobile phase. The detector response and flow of the mobile phase were monitored at 240nm and 0.5ml/min, respectively. The linearity plot was made in the concentration range of 0.1-3.0 µg/ml for metformin and 0.05-1.5 µg/ml for ertugliflozin with correlation coefficient value of more than 0.999. The developed method was subjected for bench-top, freeze and thaw, long-term and short-term stability studies and the drug components were stable over the respective conditions. The Lower limit of quantification (LLOQ) for ertugliflozin and metformin were 0.05 and 0.1 µg/ml, respectively. The findings of precision and accuracy were present in between 2.6 to 4.2 %RSD and -2 to 3.99 %RE, respectively. The findings of the stability data were presented below. The %stability of ertugliflozin and metformin were varying from 96% to 104% for ertugliflozin and 96% to 105% for metformin.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.