Abstract
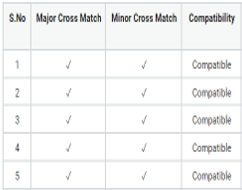
Centrifugation is a frequently employed technique in transfusion medicine used for compatibility testing, ABO and Rh typing and cross-matching. However, there is considerable evidence that it can lead to the generation of aerosol particles of size up to 5 μm. If the fluid being processed contains an infectious micro-organism like 2019 novel coronavirus (2019-nCoV), there could be a hazard due to the aerosols in the laboratory with particles capable of penetrating and being retained in the lower respiratory tract. Considering this, we wanted to determine if reliable cross-match testing can be done without the use of a centrifuge and if there are safer alternative testing methodologies available to perform a compatibility test. This was a cross-sectional study that included blood samples received in the blood bank along with requisition of blood components from 30 patients with coronavirus disease who tested 2019-nCoV positive by PCR and were admitted at BPS GMC, Sonepat and SGT Medical College Gurgaon from May 2020 to June 2020. Of the 30 samples processed, ten samples were subjected to cross-match by Immediate Spin Cross Match Method. Rest 20 samples were cross-matched by Saline Cross Match Method. However, in comparison to a standard saline cross-match where the incubation period is generally of 30 min, a prolonged incubation period of 45 min was adopted followed by addition of 22% Albumin. After that, the sample was observed for hemolysis and agglutination. The results obtained from both the methods were noted, compared and statistically analysed.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.