Abstract
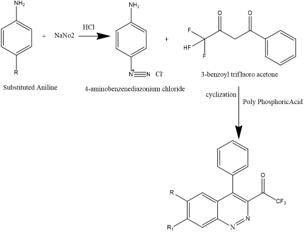
Fourteen Novel cinnoline library compounds were designed, synthesized through a facile approach, and allowed for screening for anti-bacterial activity and anti-tubercular activity. The titled compounds were entirely synthesized by replacing alkyl groups, sulphonyl, halo groups in the 6th & 7th position of cinnoline moiety. The enlightenment of structure was done by FTIR HNMR along with elemental analysis and further docked for Structural activity. The newly synthesized Cinnoline Compounds were examined for their in vitro drug-sensitive M tuberculosis H37Hv strain. All the compounds have shown MIC between >100-12.5 μg /ml. In this investigation, we Evaluated all the compounds for Anti-bacterial activity. The main compounds were initially tested in vitro for Anti-bacterial activity against gram-positive and gram-negative bacteria by using the Disk plate method. The most active Compound 10 exhibited 12.5 μg /ml inhibitions against drug-sensitive M Tuberculosis H37Rv strain. Among all synthesized compounds CN-7 was found to be a Hit compound with MIC value 12.5 ug/ml Against E Coli.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.