Abstract
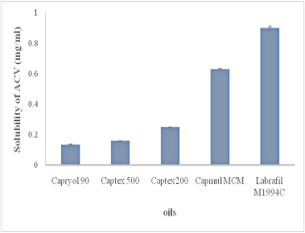
The purpose of the present study was to formulate and evaluate microemulsion based in situ gel of Acyclovir (ACV) for the vaginal delivery. The solubility of ACV in oils and surfactants and co-surfactant was evaluated to identify the components of the microemulsion. Microemulsion region was determined by using the pseudo-ternary phase diagrams for different formulations. Microemulsion formulation was prepared using Labrafil M1994C as oil phase, Cremophor RH40 as surfactant and Polyethylene glycol 400 and Transcutol P as co-surfactant and water. Microemulsion formulations were evaluated for pH, viscosity, conductivity and stability study. In situ gel of ACV, microemulsion was prepared using thermosensitive polymer, poloxamer.In situ gelwas characterized for viscosity, gelling temperature, the effect of dilution on gelling temperature, gelling ability, and in vitro drug release and release kinetics. The globule size of developed microemulsion was less than 100 nm with PDI in the range 0.307 to 0.641. The optimized microemulsion based in situ gel demonstrated shear thinning behaviour, the gelation temperature with and without dilution was in the range of 30-35ºC, and the drug release was sustained over eight hours. Mucoadhesive properties of microemulsion based in situ gel formulations were determined with a texture analyzer using a goat vaginal tissue, and the results indicated that the presence of microemulsion increased the mucoadhesion significantly. Microemulsion based in situ gel was successfully developed for vaginal delivery of Acyclovir.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.