Abstract
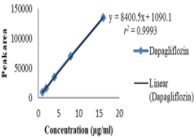
The present study is aimed to develop a linear, precise and accurate RP-HPLC (Reverse Phase High-Performance Liquid Chromatography) method for the determination of dapagliflozin in the formulation. The method was accomplished on a C18 column (250×4.6mm; 5µm), & Samples were eluted using acetonitrile: water (40:60%v/v) delivered at a flow rate of 1.0ml/min with a chromatographic run time of 10 min. The eluents were observed utilizing a UV detector with a wavelength set at 277nm. The method that was developed resulted in the retention of dapagliflozin at 7.029minutes. Dapagliflozin through current method has shown linearity (r2 > 0.999) over the concentration range of 1-16 µg/ml. The percentage recovery was observed to be within the limits of 98-102%, demonstrating the accuracy of the method. Limit of detection (LOD) and limit of quantification (LOQ) were qualified at 0.049µg/ml and 0.1485µg/ml, respectively. A Linear precise, accurate, simple, and rapid RP-HPLC method has been developed and validated for the evaluation of dapagliflozin in bulk drug and tablet dosage forms (5mg &10mg) according to ICH Q2(R1) rules. Additionally, the proposed method could be of use in quality control tests of dapagliflozin in pharmaceutical industries.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.