Abstract
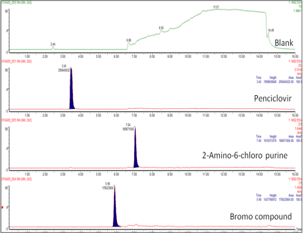
A selective, rapid and sensitive method was developed for the determination of genotoxic impurities (2-Amino-6-chloro purine and Bromo compound) in Penciclovir drug substance using RPUPLC-MS/MS. The chromatographic separation was performed on Kromasil C8 column (150 mm x 4.6 mm, 5 μm) maintained at 45°C using 0.1%formic acids in water as buffer and acetonitrile through gradient programme. The flow rate was maintained at 0.5mL/min with an injection volume of 10 μL. For the quantification of genotoxic impurities, positive-electrospray ionisation (ESI) mode was selected. Penciclovir and its impurities were well separated within the shortest run time of 16min. The chromatographic method was developed, and the results of all validation parameters showed that the technique is well confined to the limits of ICH guidelines. The method has high sensitivity, and the limit of detection was found to be as low as 0.15 and 0.30 ppm for 2-Amino-6-chloro purine and Bromo compound. The recovery of 2-Amino-6-chloro purine and Bromo compound are found in the range of 80-120%. The linearity of peak area versus concentration was demonstrated in the range of LOQ - 150% level of impurities with a correlation coefficient of 0.9999. The method has proved too robust by introducing minuscule changes in the chromatographic parameters. The method was successfully validated and applied for Penciclovir drug substances and their dosage forms to determine the mentioned genotoxic impurities.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.