Abstract
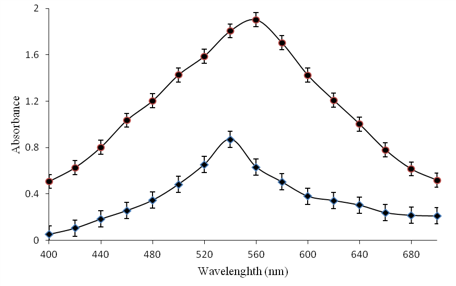
Nanoparticles of enzyme Nitrate reductase (NaR) a soluble homodimer enzyme of 100 kDa polypeptide with cofactors – FAD, heme-molybdopterin (Mo-MPT) and electron donor NAD(P)H, catalyses the reduction of nitrate to nitrite has been synthesised. Nanoparticles of Nitrate reductase enzyme have been prepared by chemical desolvation method including glutaraldehyde cross-linking to form the nanoaggregate. Characterisation of NaR nanoparticles has been made by Transmission Electron Microscopy (TEM), UV-Visible Spectroscopy and by electrochemical Impedance Spectroscopic Study (EIS). TEM study revealed the size of globular aggregated was in the range of 20–30 nm. UV Visible spectroscopic studies depicted that the absorption of NaR NPS is much higher at 560 nm than that of the free enzyme, which showed maximum absorption at 540 nm. NaR NPs aggregates formed were more active, highly stable, have a higher shelf life and can be reused repeatedly. Enzyme nanoparticles with 10-100 nm dimensions and exhibit unique physical, chemical and catalytic properties due to increased surface area. Nitrate reductase nanoparticles can be used as a biochemical tool to increase protein production and grain yield by promoting amino acids production in plants. The synthesised NaR NPs are used in the fabrication of enzyme-based nanosensor in the detection of nitrates in drinking water and serum samples.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.