Abstract
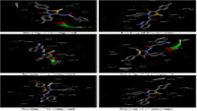
The multifarious metabolic syndrome, diabetes mellitus (DM), is a diseaseof concern all over the world and is approximate to affect 400 million individuals by the 2020. Several classes of drugs at the moment are available to lessen hyperglycemia in diabetes mellitus especially in Type-II. These drugs mostly have dangerous side effects and thus incisive for a new class of compounds is necessary to conquer this inconvenience. A series of 6 novel 5-nitrobenzofuran-2yl-carbamides derivatives were synthesized and molecular docking studies were performed on PPAR-γ target using (PDB code-4rfm).The preparation of5-nitro-1-benzofuran-2-carbohydrazide(4) on action with acetic acid, 1, 4-diaxone and sodium nitrite resulted in 5-nitro-1-benzofuran-2-carbonyl azide (5).The related compound (5) on action with substituted aromatic substituted amines undergoes Curtis type of rearrangement to give 5-nitro-N-(sub. carbamoyl)-1-benzofuran-2-carboxamide.The characterization and identification of prepared compounds were identified on the basis of NMR, IR, Mass and elemental analysis. Docking study of targeted compounds were done using software Autodock Tools 1.5.6 and visualisation done by Discovery Studio 3.5 software (Accelrys Inc. San Diego, CA USA). Molecular docking studies, the binding energies are determined to be in the range of –5.90 to –9.80 kcal/mol, with peroxisome proliferator activated receptor γ (PPAR-γ) receptors (PDB ID: 4RFM). The prepared compounds have been studied for their oral glucose tolerance test to distinguish the effect on plasma glucose level.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.