Abstract
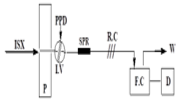
A reversed flow injection system using a solid phase reactor was developed for spectrophotometric determination of isoxsuprine hydrochloride (ISX) in bulk and pharmaceutical tablets. The reactor (4 i.d. and 40 mm length) packed with PbO2 immobilized in a polymeric matrix (150 mg) was used to oxidized p-phenylenediamine to quinone diimine which is transported to the liquid phase and subsequently coupled with ISX to produce a green product whose absorbance was monitored spectrophotometrically at 690 nm. All the physical and chemical parameters that affected the flow injection manifold were studied carefully. In addition, the variables of the reactor involved the chemical composition, degree of packing, the reactor length and particle size were also optimized. The calibration graph for ISX was linear over the range of 25–300 µg/mL with the detection limit of 8.96 of ISX. The solid phase material was stable for more than one month and the relative standard deviation was best than 2% with the sampling frequency of 36 samples/hour. The reaction stoichiometry was evaluated by Job’s method and was found to be 1:1 (ISX: PPD). The method was applied for the assay of ISX in pharmaceutical samples and the results obtained are an agreement with those obtained using the standard Pharmacopeia method.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.