Abstract
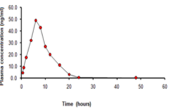
Chrono pharmaceutical drug delivery system is devoted to the availability of active on a pace that idyllically matches biological requisite of disease therapy. It embodies time controlled and site-specific drug delivery systems, subsequently optimizing therapeutic action and lessening side-effects. In the present study, the optimized pulsatile formulation of Propranolol Hydrochloride ought to deliver a drug at the pre-set pattern at the right time and site is evaluated for pharmacodynamic and pharmacokinetic performance after oral administration and compared with an existing marketed sustained formulation of Propranolol HCl. The study was carried out in male New Zealand albino rabbits through the cross over design pattern and levels of plasma measured by means of LC-MS/MS method. Pharmacokinetic parameters of designed pulsatile formulation were measured and observed to have statistical significance with the existing marketed sustained formulation. The In-house pulsatile dosage form able to show the lag phase and the mean residence time of pulsatile dosage form (23.20.14h.) was found to be beyond the marketed sustained dosage form (14.8 0.01h.). Pharmacodynamic data revealed a maximum guard against adrenaline levels at 6 h post-oral intake and dropped to 50% after 12 h with Marketed formulation. Whereas in the case of pulsatile formulation administration, maximum protection was obtained at 12 h. and continued over a period of 18 h. It is concluded that the designed pulsatile formulation offers a promising way of drug release for a programmed time period at the desired site, once the administration time and pulse time are aligned with a circadian pattern.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.