Abstract
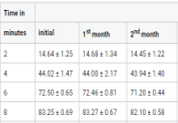
Solid dosage forms also have a impervious difficulties in patients especially for geriatric and paediatric patients.Dysphagia is common among all age groups. Orodispersible formulations (Fast dissolving tablets & Fast dissolving oral thin films) constitute an inventive dosage form that overcome the problems swallowing and provides speedy onset of action. The objective of present study was to formulate orodispersible formulations of Hydralazine HCL by different methods (Direct compression method, Sublimation method and solvent casting method). Based on physiochemical evaluations F9 (Direct compression method),SF9 (Sublimation method) for Fast dissolving tablets and H2 formulations (Solvent casting method) for Fast dissolving oral thin films were found optimized formula. The optimized formula were kept for stability under long term, accelerated and intermediate conditions for the study period of six months as per ICH guidelines.Based on stability reports the H2 formulations (Fast dissolving oral thin films) got a better drug release than Fast dissolving tablets.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.