Abstract
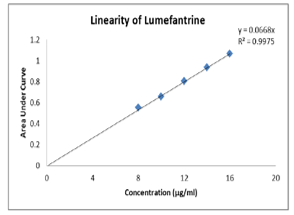
A Simple, sensitive, specific, spectrophotometric method has been developed for the detection of Lumefantrine in pure form and Pharmaceutical formulations. The optimum condition for the analysis of the drug was established. Lumefantrine exhibiting absorption at 234nm and obeyed beers law in the concentration range 8 to 16μg/ml. The lower limit of detection was found to be 4.3×10-2 and the limit of quantification to be 13.2 ×10-2. The regression equation was y = 0.065x + 0.02. The precision of the method was found to be 480.96mg at 234nm against the label claim of 480mg. The sample solution was stable up to 24 hours. The assay results were found to be in good agreement with label claim. The proposed method was simple sensitive, precise, quick and useful for routine quality control.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.