Abstract
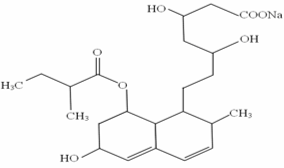
A simple RP-HPLC method for the determination of pravastatin in tablet dosage form. Numerous HPLC conditions were tested for the determination of pravastatin. The best result was achieved by using Phenomenex® Luna 5μm C18( 150x4.6mm) ID column, and a mobile phase consisting of acetonitrile: potassium dihydrogen orthophosphate (0.02M) (30:70) adjusted to pH 3.0 with orthophosphoric acid, a flow rate of 1.5ml/min with ultraviolet detection at 240nm.The correlation coefficient for calibration curves within the detection range of 35.22-65.40μl/ml are 0.9993. The within and between-day precision was determined for both retention time and peak area.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.