Abstract
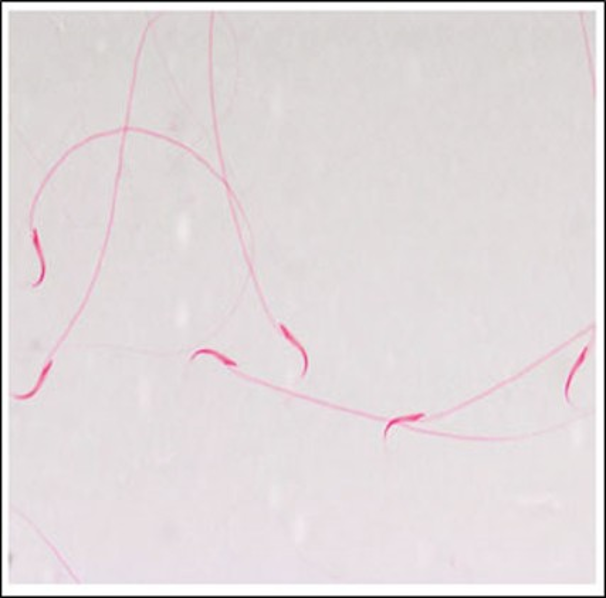
Cancer treatments can affect sperm production and a significant percentage of cancer patients may develop permanent azoospermia or severe oligozoospermia after chemotherapy. To investigate the influence of Gemcitabine toxicity on the reproductive system of albino male rats (sperm count and morphology). An experimental animal study conducted in the zoology department, College of Science, King Saud University during the period from June to October 2014 using albino rats (Rattusnorvegicus) (Wistar strain). Males were divided into four different groups (control” 0 mg/kg”,7 mg/kg,14 mg/kg, and 21 mg/kg). The reproductive organs, testicles and epididymis decreased in weight and atrophied in most of the animals treated with the drug in various doses. The mean absolute and relative epididymal weights were also significantly decreased. In the drug-effects recovery group, neither the testicles nor the epididymis in the animals treated with the three doses recovered fully normal weight. The testis’s efficiency in producing sperm was significantly decreased at all doses. In the recovery group, the testis regained its efficiency, as no significant difference was recorded between the drug-treated groups and the control group. The drug caused complete loss of sperm, in a rat treated with the big dose. Gemcitabine caused a significant increase in the percentage of deformed sperms in all treated animals. Gemcitabine drug has high toxicity on the reproductive system of rats with a dose tenth of human dose, with a massive decrease in the count and quantity of sperm, which means that this drug can have more toxicity effects on human.
Full text article
References
AdisInsight 2006. Gemcitabine - Eli Lilly and Company/Genentech. Eli Lilly and Company, Accessed on: 25 Oct 2020.
Amann, R. P. 1981. A Critical Review of Methods for Evaluation of Spermatogenesis from Seminal Characteristics. Journal of Andrology, 2(1):37–58.
Amann, R. P. 1986. Detection of alterations in testicular and epididymal function in laboratory animals. Environmental Health Perspectives, 70:149– 158.
Andrade, A. J., Araújo, S., Santana, G. M., Ohi, M., Dalsenter, P. R. 2002. Reproductive Effects of Deltamethrin on Male Offspring of Rats Exposed during Pregnancy and Lactation. Regulatory Toxicology and Pharmacology, 36(3):310–317.
Brougham, M. F. H., Kelnar, C. J. H., Sharpe, R. M., Wallace, W. H. B. 2003. Male fertility following childhood cancer: current concepts and future therapies. Asian Journal of Andrology, 5(4):325–337.
Casciato, D. 2004. Manual of clinical oncology. (5th ed.). Lippincott Williams & Wilkins. ISBN: 9780781747417.
Chan, P. T. K. 2013. Fertility after cancer in men. Canadian Urological Association Journal, 3(3):223.
Chandra, A. K., Ghosh, R., Chatterjee, A., Sarkar, M. 2007. Effects of vanadate on male rat reproductive tract histology, oxidative stress markers and androgenic enzyme activities. Journal of Inorganic Biochemistry, 101(6):944–956.
Chen, J. J. 2006. Statistical Analysis for Developmental and Reproductive Toxicologists. Developmental and Reproductive Toxicology: A Practical Approach, 2nd ed. (R. D. Hood Ed.),. ISBN: 9780849312540.
Clegg, E. D., Perreault, S. D., Klinefelter, G. R. 2008. Assessment of male reproductive toxicology. Principles and methods of toxicology, pages 1263– 1299.
Creasy, D. M. 2001. Pathogenesis of Male Reproductive Toxicity. Toxicologic Pathology, 29(1):64–76.
Foster, P. M. D., Harris, M. W. 2005. Changes in Androgen-Mediated Reproductive Development in Male Rat Offspring Following Exposure to a Single Oral Dose of Flutamide at Different Gestational Ages. Toxicological Sciences, 85(2):1024–1032.
Hryciuk, B., Szymanowski, B., Romanowska, A., Salt, E., Wasąg, B., Grala, B., Jassem, J., Duchnowska, R. 2017. Severe acute toxicity following gemcitabine administration: A report of four cases with cytidine deaminase polymorphisms evaluation. Oncology Letters, 15(2):1912–1916.
Meistrich, M. L. 1986. Critical Components of Testicular Function and Sensitivity to Disruption. Biology of Reproduction, 34(1):17–28.
Meistrich, M. L. 2013. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertility and Sterility, 100(5):1180–1186.
Mini, E., Nobili, S., Caciagli, B., Landini, I., Mazzei, T. 2006. Cellular pharmacology of gemcitabine. Annals of Oncology, 17:v7–v12.
Okada, K., Fujisawa, M. 2019. Recovery of Spermatogenesis Following Cancer Treatment with Cytotoxic Chemotherapy and Radiotherapy. The World Journal of Men’s Health, 37(2):166.
Pectasides, D., Pectasides, E., Papaxoinis, G., Skondra, M., Gerostathou, M., Karageorgopoulou, S., Kamposioras, C., Tountas, N., Koumarianou, A., Psyrri, A., Macheras, A., Economopoulos, T. 2009. Testicular Function in Poor-Risk Nonseminomatous Germ Cell Tumors Treated With Methotrexate, Paclitaxel, Ifosfamide, and Cisplatin Combination Chemotherapy. Journal of Andrology, 30(3):280–286.
Ramesh, A., Inyang, F., Lunstra, D. D., Niaz, M. S., Kopsombut, P., Jones, K. M., Hood, D. B., Hills, E. R., Archibong, A. E. 2008. Alteration of fertility endpoints in adult male F-344 rats by subchronic exposure to inhaled benzo(a)pyrene. Experimental and Toxicologic Pathology, 60(4-5):269–280.
Sabanegh, E. S., Ragheb, A. M. 2009. Male Fertility After Cancer. Urology, 73(2):225–231.
Siegel, R. L., Miller, K. D., Jemal, A. 2016. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians, 66(1):7–30.
Takahashi, K. L., Takahashi, N., Hojo, H., Kuwahara, M., Aoyama, H., Teramoto, S. 2006. Pathogenetic transition in the morphology of abnormal sperm in the testes and the caput, corpus, and cauda epididymides of male rats after treatment with 4,6-dinitro-o-cresol. Reproductive Toxicology, 22(3):501–507.
Trost, L. W., Brannigan, R. E. 2012. Oncofertility and the Male Cancer Patient. Current Treatment Options in Oncology, 13(2):146–160.
Yu, G., Liu, Y., Xie, L., Wang, X. 2009. Involvement of Sertoli cells in spermatogenic failure induced by carbendazim. Environmental Toxicology and Pharmacology, 27(2):287–292.
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.