Abstract
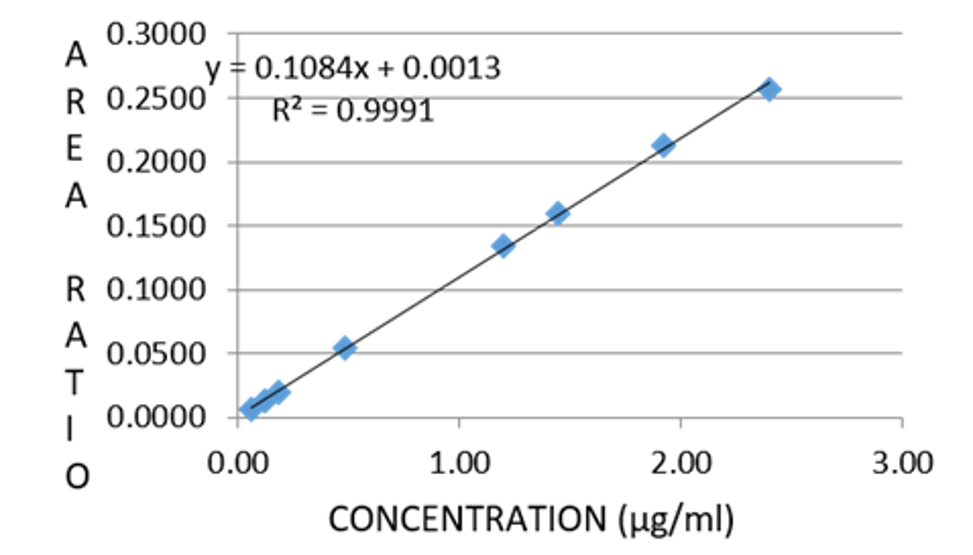
A simple, precise and accurate method was developed for the quantitative estimation of Canagliflozin in human plasma using Dapagliflozin as internal standard by Reverse Phase-High Performance Liquid Chromatographic technique. Chromatographic conditions were of stationary phase Phenomenex Luna C18 (2) (150 x 4.6 mm, 5m), Mobile phase 0.01 N Potassium dihydrogen Phosphate buffer pH (3.5 ± 0.05) : acetonitrile in the ratio of 45:55 and flow rate at 1.0 ml/min, detection wave length was UV 222 nm, column oven temperature was maintained at 30oC, and sample injection volume of 10 µL. Retention time of Canagliflozin was found to be about 8.7 min. Coefficient of Variation for Canagliflozin peak was 3.15 %, % recovery was 94.58 %. The linearity of method was studied from 0.06 µg/ml to 2.4 µg/ml (R2 = 0.999). The Signal to Noise ratio of lower limit of quantification (0.06 µg/ml) was found to be 50. The proposed bio-analytical method was validated by following ICH guidelines.
Full text article
References
Devineni, D., Polidori, D. 2015. Clinical Pharmacokinetic, Pharmacodynamic, and Drug–Drug Interaction Profile of Canagliflozin, a Sodium-Glucose Cotransporter 2 Inhibitor. Clinical Pharmacokinetics, 54(10):1027–1041.
D’souza, M. S., Krishna, Gude, S., Sushmitha, Vas- antharaju, S. G. 2016. Stability Indicating Assay Method Development and Validation to Simultaneously Estimate Metformin Hydrochloride and Canagliflozin by RP-HPLC. Curr. Trends Biotechnol. Pharm, 10(4):334–342.
Elflien, J. 2019. Diabetes - Statistics and Facts. Inzucchi, S. E., Bergenstal, R. M., Buse, J. B., Diamant, M., Ferrannini, E., Nauck, M., Peters, A. L., Tsapas, A., Wender, R., David, R. 2012. Matthews Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Diabetes Care, 35(10):1364–1379.
Kaur, I., Wakode, S. 2017. Harsharan Pal Singh, Development and Validation of a Stability- Indicating High Performance Thin Layer Chromatography (HPTLC) Method for estimation of Canagliflozin in bulk and Pharmaceutical Dosage Form. J App Pharm Sci, (5):51–057.
Kaur, I., Wakode, S., Singh, H. P., Manachanda, S. 2016. Development and Validation of a Stability- Indicating Reverse Phase HPLC-PDA Method for Determination of Canagliflozin in Bulk and Pharmaceutical Dosage Form. Pharmaceutical Methods, 7(1):54–62.
Krishna, V. R., Nalini, A. S., N, C. 2018. Validation of a Stability Indicating Reverse Phase HPLC method for determination of canagliflozin API. World Journal of Pharmaceutical Research, 7(3):459–468.
Ladva, B. J., Dobariya, P. V., Pancholi, H. D., Nayak, B. S., Jain, S., Rp 2016. HPLC Development and Validation of Chromatographic Method for Estimation of Canagliflozin in API and Tablet Dosage Form. Int. J. Recent Sci. Res, 7(5):10976–10979.
Marella, A. V. L., Syed, L., Prasanna 2017. Buchi Naidu Nalluri, A novel validated RP-HPLC method for the estimation of canagliflozin in bulk and pharmaceutical dosage forms. Int. j. adv. pharm, 7(03):24–27.
Pooja, C., Singh, G., Sisodiya, D. 2018. Development and Validation of UV Spectrophotometric Method for the Estimation of Canagliflozin in Bulk and Pharmaceutical Dosage Form. Int. j. pharm, 13(1):1–9.
Saibaba, N. S. V., Pilli, B. P. K. R., Bimireddy 2018. Pitchaimuthu Shanmuga Pandiyan, A novel and rapid LCeMS/MS assay method for the determination of canagliflozin in human plasma by solid phase extraction technique and its application to a pharmacokinetic study. Future Journal of Pharmaceutical Sciences, 4:131–138.
Sen, G., Babu, K. R., Annapurna, N., Vekariya, N. A., Kumar, V. J., Kumar, K. S. R. P., Sharma, H. K. 2018. Validation of Stability-Indicating Reverse Phase HPLC Method for the Determination of Related Substances in Dapagliflozin Drug Sub- stance. American Journal of PharmTech Research, 8(5):322–336.
Sreenivasulu, P., Pendem, C., Viswanadham, N. 2014. Nanoparticles of ZrPO4 for green catalytic applications.
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.