Abstract
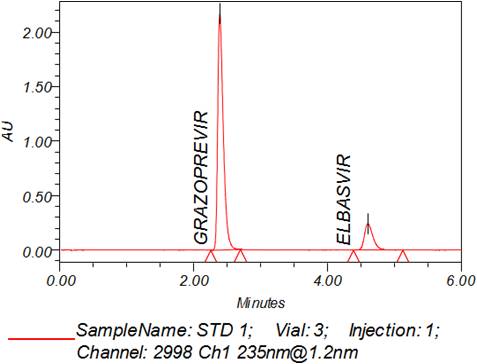
A simple, rapid and sensitive isocratic RP-HPLC method was developed for the simultaneous estimation of Grazoprevir and Elbasvir in bulk and their Pharmaceutical dosage form using Waters C18 (250 x 4.6 mm x 5 μ particle size) analytical column in an isocratic mode with mobile phase comprising Acetonitrile: 0.25M Potassium dihydrogen orthophosphate buffer (pH 4.5) (55:45, v/v). The flow rate was 1.5 ml/ min and effluent was monitored at 235nm. The retention times were found to be 2.390 min for Grazoprevir and 4.603 min for Elbasvir. The assay exhibited a linear dynamic range of 100 - 300 μg/ml for Grazoprevir and 50-150 μg/ml for Elbasvir. The calibration curve was linear (r2 = 0.9998 for Grazoprevir and r = 0.9999 for Elbasvir) over the entire linear range. Recovery was found to be 99.03% for Grazoprevir and 99.34 % for Elbasvir. The proposed method was statistically evaluated and validated as per ICH guidelines and can be applied for routine quality control analysis of Grazoprevir and Elbasvir in Pharmaceutical dosage form.
Full text article
Generated from XML file
Authors
Harshalatha P, Kothapalli Bannoth Chandrasekhar, & Chandrasekhar MV. (2018). Stability indicating RP-HPLC method for simultaneous determination of Grazoprevir and Elbasvir in bulk and tablet dosage form. International Journal of Research in Pharmaceutical Sciences, 9(4), 947–955. Retrieved from https://ijrps.com/home/article/view/4390
Copyright (c) 2018 International Journal of Research in Pharmaceutical Sciences

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.