Abstract
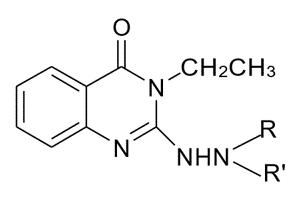
A variety of novel 3-ethyl-2-substitutedamino-quinazolin-4(3H)-ones were synthesized from 3-ethyl-2-hydrazino quinazolin-4(3H)-one with a variety of aldehydes and ketones. When tested for their in vitro antitubercular activity using H37RV strain on Middle brook 7H11 agar slants with OADC growth supplement, all the test compounds inhibited the growth of Mycobacterium tuberculosis at micro gram concentration. Among the test compounds, 2-(N-(4- Chloro-benzylidene-hydrazino)-3-ethyl-3H-quinazolin-4-one (S6) and 2-(N-(4-Nitro-benzylidene-hydrazino))-3- ethyl-3H-quinazolin-4-one (S7) are found to be the most active compounds against M.tuberculosis with the MIC of 6µg/ml. The title compounds are also screened for the antimicrobial activity against some other gram positive and gram negative bacteria by agar dilution method, compounds S6 and S7 showed the most potent activity (MIC in the range of 32-63 µg/ml) against the tested bacteria.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.