Abstract
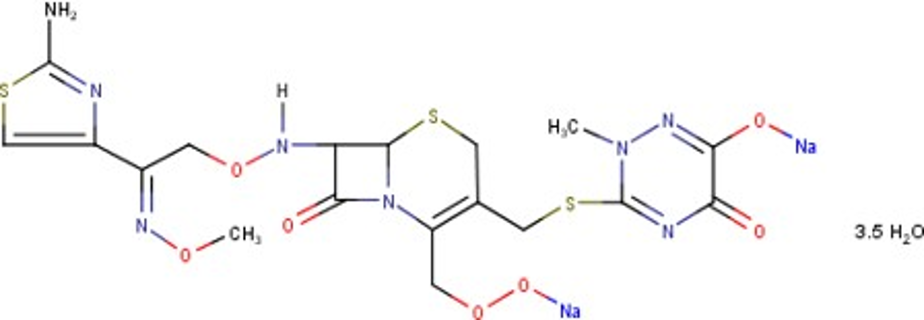
The use of the ceftriaxone in the treatment of infections of the urinary tract and respiratory tract associated with sepsis both intravenously and intramuscularly is the primary choice in therapy. To support drug therapy monitoring (TDM), a simple, fast, inexpensive and validated analysis method is needed. The availability of UV-Vis spectrophotometer in almost all clinical laboratories is high, so it can be an alternative to apply in TDM of patients. Accuracy, inter-and intra-day precision, linearity, freeze and thaw stability, and short and long-term stability were parameters determined. The maximum wavelength of ceftriaxone was 272 nm, with linearity at the concentration range of 10-30 µg/mL with a correlation coefficient (r2) of 0.99. The accuracy of the method was studied by recovery study. The result showed that % recovery was found in the range of 94-99% with relative standard deviation (RSD) less than 2%. Intra and inter-day precision were accepted with RSD value of < 2%. The spiked human plasma sample containing ceftriaxone remained stable for 4 hours at room temperature and was stable in storage at -20 ◦C for seven days. The method is simple, accurate and inexpensive. The proposed method can be successfully used for the determination of Ceftriaxone sodium in human plasma.
Full text article
Authors

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.