Role of GS-5734 (Remdesivir) in inhibiting SARS-CoV and MERS-CoV: The expected role of GS-5734 (Remdesivir) in COVID-19 (2019-nCoV) - VYTR hypothesis
Abstract
Coronaviruses (CoVs) are enveloped RNA viruses related to the family Coronaviridae, the order Nirdovales, and observed in humans and other mammals. In December 2019, many pneumonia cases reported by patients with unknown causes, mainly associated with seafood and wet animal market in Wuhan, China, and where clinically resembled viral pneumonia. At present, there is no existence of antiviral drugs for the treatment of CoV infections. The results of our study are GS-5734 strongly inhibits SARS-CoV and MERS-CoV in HAE cells, GS-5734 inhibits CoVs at early stages in replication by inhibiting viral RNA synthesis, the absence of ExoN-mediated proofreading in viruses sensitive to treatment with GS-5734. Protease inhibitors can show improved outcomes in some coronaviruses, but mostly 99% of protease inhibitors bind to proteins present in the human body, and only 1% attacks on existed viruses. The expected role of GS-5734 (Remdesivir) in COVID-19 (2019-nCoV) - VYTR hypothesis explained. As broad-spectrum drugs are capable of inhibiting CoV infections, GS-5734 is a broad-spectrum drug and may show inhibition on CoV infections and COVID-19. GS-5734 will show desired results regarding antiviral activity against 2019-nCoV as it showed potent antiviral activity in other CoVs. More clinical trials and experiments needed to prove that GS-5734 (Remdesivir) is a potential and effective drug to treat COVID-19.
Keywords
Remdesivir, GS-5734, VYTR hypothesis, SARS-CoV, MERS-CoV, 2019-nCoV (2019 novel coronavirus), Anti-viral drugs
Introduction
Coronaviruses (CoVs) are enveloped RNA viruses related to the family Coronaviridae, the order Nirdovales, and observed in humans and other mammals (Richman, Whitley, & Hayden, 2016). Six coronavirus species are cause for disease in humans. Prevalent four viruses are 229E, OC43, NL63, and HKU1 cause common cold symptoms in immunocompetent people (Su et al., 2016). Remaining two viruses are severe acute respiratory syndrome coronavirus (SARS-CoV) and Middle East respiratory syndrome coronavirus (MERS-CoV), zoonotic in origin, and cause fatal illness sometimes (Su et al., 2016). CoVs causes cold and pneumonia before SARS-CoV in 2002 and MERS-CoV in 2012 from zoonotic sources (Ksiazek et al., 2003; Zaki, Boheemen, Bestebroer, Osterhaus, & Fouchier, 2012). In December 2019, many pneumonia cases reported by patients with unknown causes, mainly associated with seafood and wet animal market in Wuhan, China, and where clinically resembled viral pneumonia (Wuhan Municipal Health Commission, 2019). The antiviral drugs, protease inhibitors such as indinavir, saquinavir, and lopinavir/ritonavir, in the research. Remdesivir, interferon beta are also examined as possible treatments.
At present, there is no existence of antiviral drugs for the treatment of CoV infections. In our study, CoV resistance to the 5-fluorouracil (5-FU) and ribavirin (RBV) in vitro is attributed to their removal by the proofreading ExoN (Smith, Blanc, Vignuzzi, & Denison, 2013), supporting this potent nucleoside analog must avoid proofreading to intervene with CoV RNA synthesis.
GS-5734 (Monophosphoramidate prodrug), inhibits SARS-CoV, MERS-CoV, and bat CoV strains which in replicating in primary human airway epithelial cells (HAE) and mediate entry using human CoV receptors (Cho et al., 2012; Sheahan et al., 2017; Warren et al., 2014).
The three objectives of our study are,
-
Role of GS-5734 in inhibiting SARS-CoV and MERS-CoV in HAE cells.
-
Role of GS-5734 in CoVs inhibition at early stages in replication by inhibiting viral RNA synthesis.
-
The expected role of GS-5734 (Remdesivir) in the 2019-nCoV (COVID-19) – VYTR hypothesis
Role of GS-5734 (Remdesivir)
Inhibition of MHV replication
To describe if GS-5734 inhibits the model B-2aCoV, in this study, delayed brain tumor (DBT) cells are infected with murine hepatitis virus (MHV). Treating with elevating concentrations of GS-5734 showed a reduced viral titer till 6-log10, and concentrations of more than 0.7 µM GS-5734 (Figure 1 (A)) did not detect the virus by plaque assay. GS-5734 strongly inhibited MHV with IC50 of 0.05 µM (Figure 1 (B)), maintained consistency with more permeability in cells, and well-organized metabolism to active triphosphate (TP) by avoiding the rate-limiting first phosphorylation step.
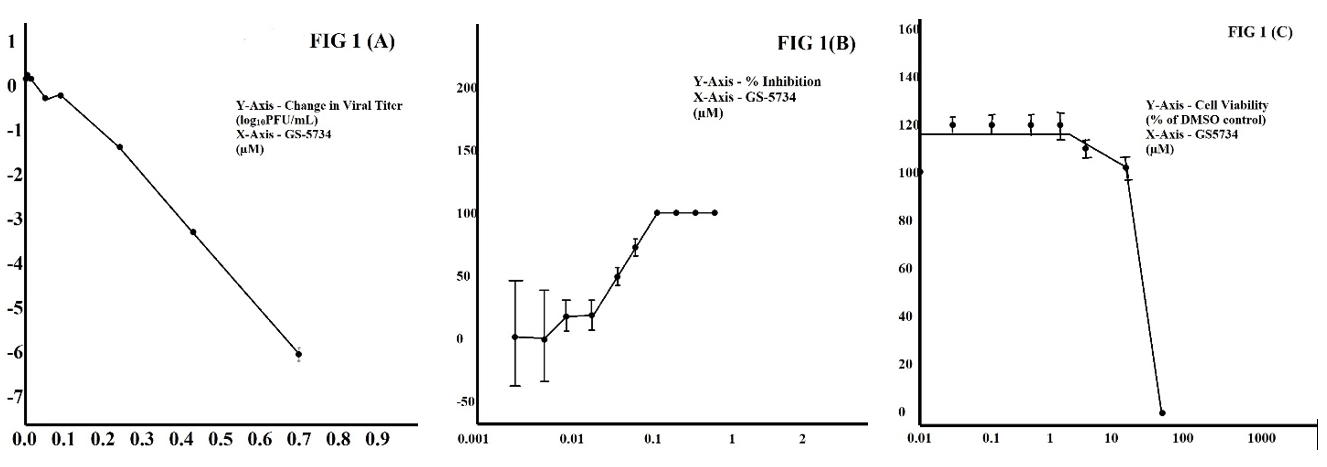
In our study, low cellular toxicity at required concentrations for antiviral activity of GS-5734, formerly delineated study of cytotoxicity extensively in human cells, with a CC50 of 41 µm (Figure 1 (C)) and showing in a selectivity index of >1000. The above results determine that GS-5734 inhibits MHV replication.
Inhibition in HAE cells
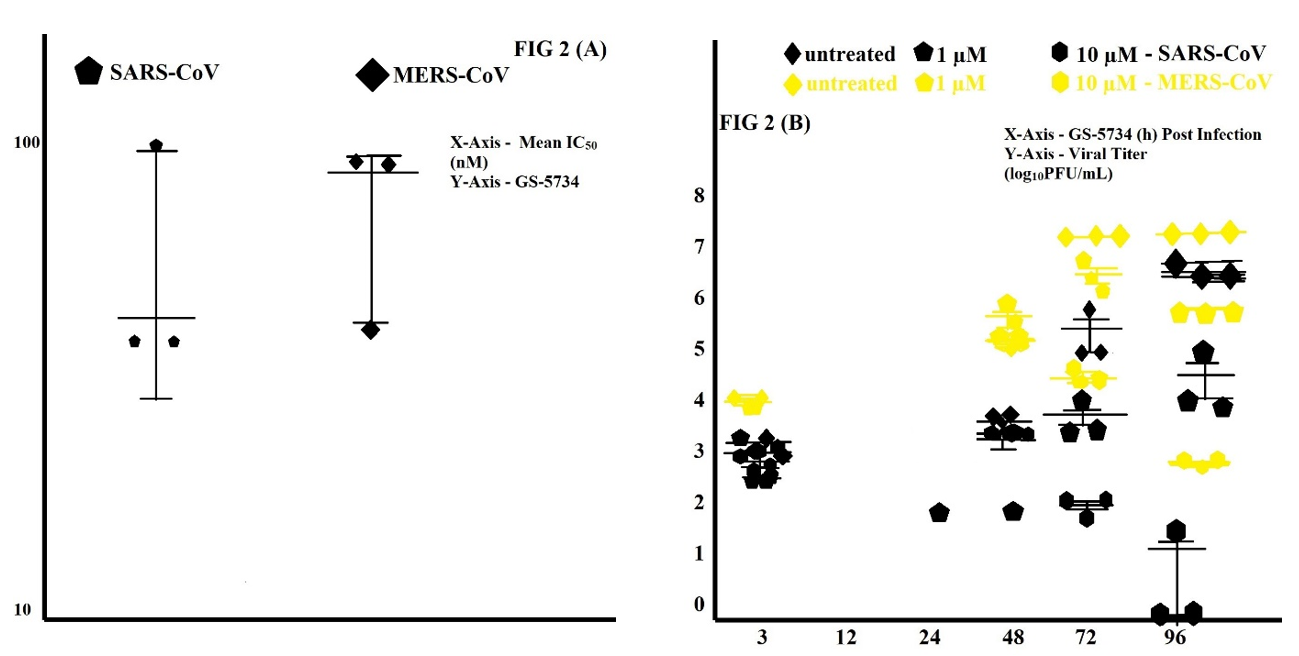
HAE cell cultures are in the biologically pertinent in vitro lung models, summarizing the complexity of cells and epithelium physiology in the human conducting airway (Sims et al., 2005). Our research study described the IC50 values after treatment with GS-5734 in SARS-CoV- infected HAE cultures and MERS-CoV-infected HAE cultures. Mean IC50 for two viruses is 0.076 µM for GS-5734 (Figure 2 (A)). Delaying the addition of GS-5734 until 48 hours (h) post-infection showed reduced viral titer in HAE cultures for SARS-CoV and MERS-CoV (Figure 2 (B)) at 72 and 96 h post-infection. Cytotoxicity in HAE cultures are not measurable (Table 1).
|
GS-5734 |
||
|---|---|---|
|
|
IC50 (µM) |
CC50 (µM) |
|
SARS-CoV |
0.071 + 0.038 |
> 10 |
|
MERS-CoV |
0.76 + 0.025 |
> 10 |
The above results determine that GS-5734 strongly inhibits SARS-CoV and MERS-CoV in HAE cells.
Inhibition of viral RNA synthesis
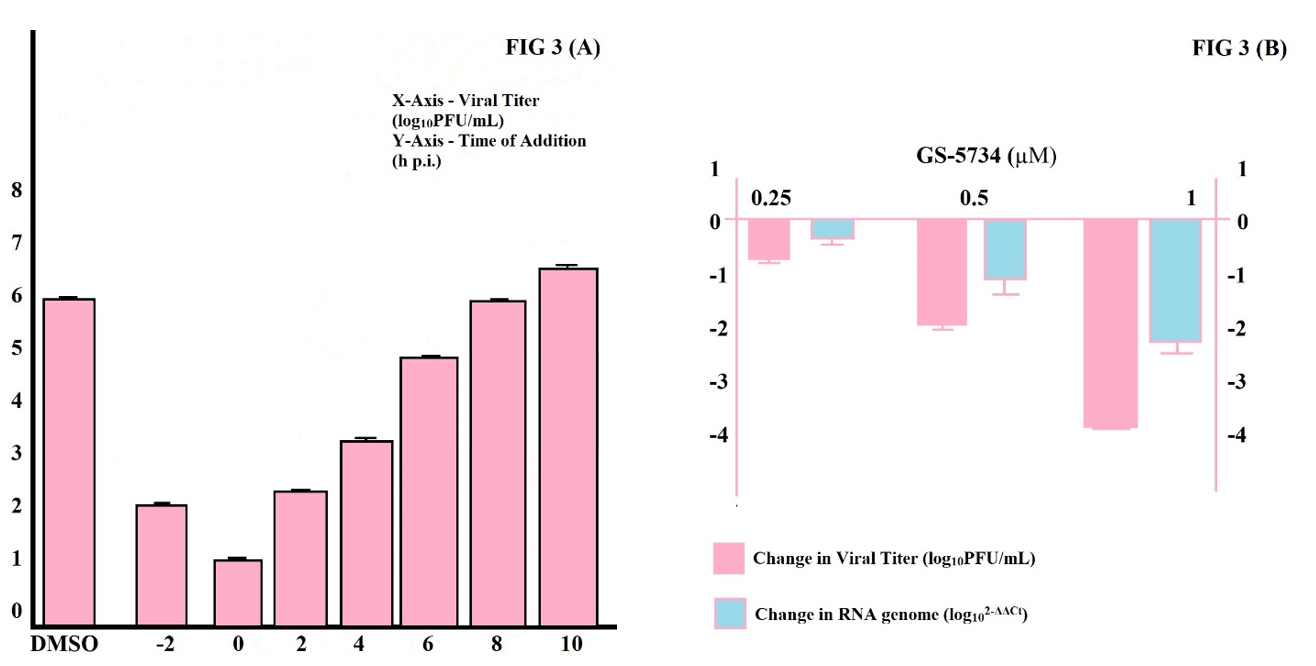
In our study, cells infected with MHV at a multiplicity of infection (MOI) of 1 PFU/cell, MHV shows in a single-cycle disease and treated with 2 µM GS-5734 at 2-h intervals from 4 h preinfection to 10 h post-infection. Our study showed, optimal inhibition of if GS-5734 included between 4 h preinfection and 4 h post-infection. Lower inhibition of GS-5734 if it was included between 6 and 8 h post-infection, and no inhibition of GS-5734 if it included after 10 h post-infection (Figure 3 (A)). This description clearly shows GS-5734 inhibits CoVs at early in infection, due to synthesis of viral RNA early in infection and GS-5734 is involved in inhibition of viral RNA synthesis (Fehr & Perlman, 2015; Warren et al., 2014), after this explained the viral RNA cellular level by real-time PCR (RT-PCR) after treatment with GS-5734. Treatment with elevating concentrations of GS-5734 showed reduced viral RNA levels associated with the reduction in titer (Figure 3 (B)).
The above results determine that GS-5734 inhibits CoVs at early stages in replication by inhibiting viral RNA synthesis.
ExoN-mediated proofreading
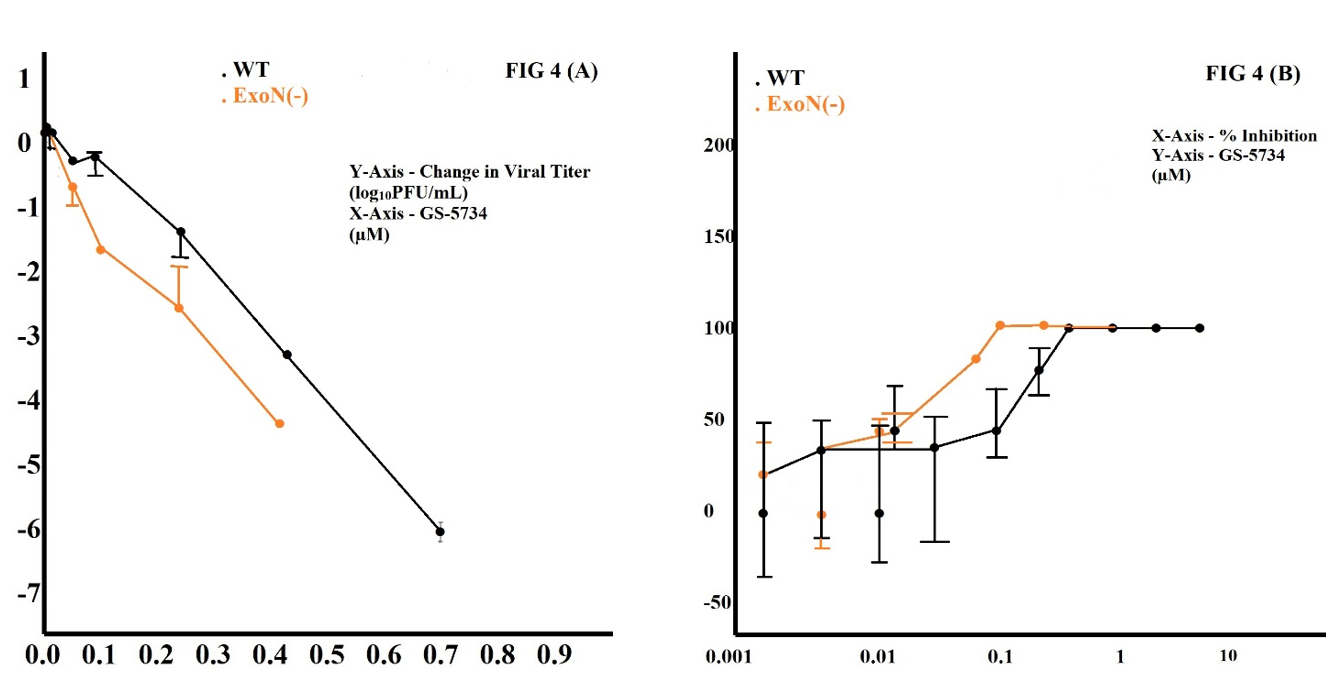
In our study, the resistance of CoVs to the RBV and 5-FU because nsp14 has proofreading ExoN, as prepared ExoN(—) mutant MHV and SARS-CoV are sensitive (Smith et al., 2013). In our study, we made a comparison of the sensitivity of WT and ExoN(—) MHV to GS-5734. ExoN(—) MHV described a 100-fold more significant reduction in viral titer at 0.27 µM GS-5734 compared to WT virus (Figure 4 (A)), and the measured IC50 for ExoN(—) virus in this study was 0.021 µM, a 4.5-fold reduced compared to the WT IC50 of 0.089 µM (Figure 4 (B)). This elevated sensitivity of the ExoN(—) virus to GS-5734 is the same as other nucleoside analogs and proposes that GS-5734 is included in viral RNA and separated by ExoN. Different associations of GS-5734 with the CoV replicase and template RNA compared with RBV and 5-FU, with intact proofreading GS-5734, inhibits CoVs (Smith et al., 2013). The mechanism is by introducing TP into viral RNA. In our study, GS-5734 acts early in infection and reduces RNA levels in a dose-dependent manner, which impairs viral titer. Results clearly explain the absence of ExoN-mediated proofreading in viruses is sensitive if treated by GS-5734. GS-5734 identified and eliminated by ExoN not in a well-organized manner, and even future studies are required to know the ExoN role in GS-5734.
Short description of anti-viral drugs on Coronaviruses
When there is a dangerous new virus emerging in humans, scientists fetch many months and years developing, testing, and perfect functioning of a drug and vaccine.
With the boutade of COVID-19, the use of anti-HIV drugs in the treatment of COVID-19 got prominence. An anti-HIV drug combination of lopinavir and ritonavir and this drug combination mainly target protease during the process of producing new copies of HIV and coronavirus slash proteins by using protease enzyme.
Protease inhibitors can show improved outcomes in some coronaviruses, but mostly 99% of protease inhibitors bind to proteins present in the human body, and only 1% attacks on existed viruses. Mainly, protease inhibitors show practical actions against HIV due to as it is having considerably more sensitivity to the drug. If it comes to coronavirus protease inhibitors shows insensitivity to the drug. It is not possible to get a free level of drugs that allow working in the human body.
Anti-viral drugs like ribavirin and lopinavir-ritonavir, immune modulators like corticosteroids and interferon treated both SARS-CoV and MERS-CoV and all didn't show desired results in a trial (Zumla, Chan, Azhar, Hui, & Yuen, 2016). Multiple cell lines showed anti-viral effects of FDA approved drugs (ribavirin and lopinavir, ritonavir, nelfinavir, and mycophenolic acid) where results and experimental issues make explanations difficult (Zumla et al., 2016).
In mouse, Prophylactic treatment and Therapeutic treatment with GS-5734 decreased virus lung titers improved pulmonary function and symptoms in SARS-CoV (Sheahan et al., 2017).
The expected role of GS-5734 (Remdesivir) in COVID-19 (2019-nCoV) - VYTR hypothesis
Genetic sequence of 2019-nCoV has resemblance with SARS-CoV (79.5%) and bat CoV (96%) (Zhou et al., 2020). 2019-nCoV related to SARS-CoV, from the subgenus Sarbecovirus (Beta-CoV lineage B) (Hui et al., 2020). In a study, the novel CoV resembled SARS-CoV by utilizing a similar cell entry receptor called ACE2 (Zhou et al., 2020).
In this study, we showed that GS-5734 was valid towards SARS-CoV and MERS-CoV, and possibly this Will have the same effect even on COVID-19. Treating in elevating concentrations of GS-5734 may show a reduction in viral titer for 2019-nCoV. Mostly, delaying the addition of GS-5734 from 24 h – 48 h postinfection may show reduced viral titer for 2019-nCoV, this result may determine that GS-5734 strongly inhibits 2019-nCoV. Treating in elevating concentrations of GS-5734 may reduce levels of viral RNA associated with the reduction of titer. As our results conclude that GS-5734 inhibits CoVs at early stages in replication by inhibition of synthesis of viral RNA and the same as in remaining CoVs, GS-5734 may inhibit even 2019-nCoV at early stages. I expect and firmly believe that GS-5734 will show desired results regarding anti-viral activity against COVID-19.
As broad-spectrum drugs are capable of inhibiting CoV infections, GS-5734 is a broad-spectrum drug and may show inhibition on CoV infections and COVID-19.
But GS-5734 (Remdesivir) is not approved and not determined to be safe or effective at present; more clinical trials and experiments needed to prove that GS-5734 (Remdesivir) is a potential and effective Drug to treat COVID-19.
Conclusions
Viral diseases can be catastrophic like COVID-19, and they may have both social and economic issues. Strict, well-organized, structured, and scheduled infection control policies should be made national and international wide. To prevent outbreaks, hospitals should be ready with control measures and protocols during handling cases. Many clinical trials and experiments needed to find effective drugs and vaccines to treat 2019-nCoV.